Latest News
Research
Massey researcher selected as national leader for large-scale NCI clinical trial
Jun 15, 2015

Massey physician-scientist Andrew Poklepovic, M.D., has been selected as a national co-primary investigator (PI) for a large-scale clinical trial led by the National Cancer Institute (NCI).
Known as the NCI-MATCH trial – National Cancer Institute Molecular Analysis for Therapy Choice Program, the study will consist of numerous small, phase 2 trials that will examine solid tumors and lymphomas that no longer respond to standard treatment and have begun to grow. Next-generation DNA sequencing will be administered on tumor biopsies from as many as 3,000 patients nationwide to determine the cell’s “broken” mechanism and, therefore, what is causing the cancer growth. Once the mutation is identified, the appropriate PI will be contacted and protocol will be directed.
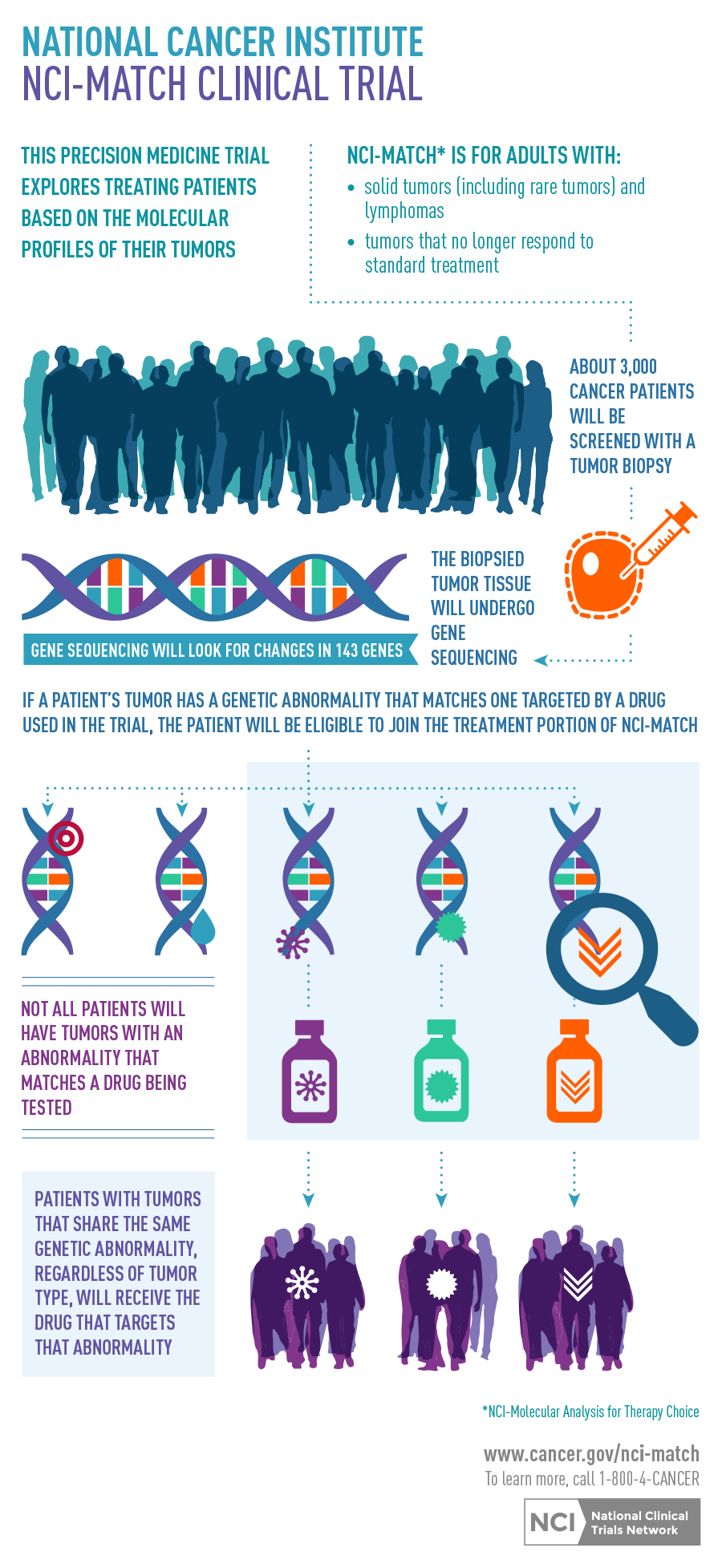
“This trial is an important step toward personalized cancer treatments for patients. It is assessing cancer pathways and how a broken pathway can lead to a cell being able to continue to grow forever and make new cells. If you can target that pathway, you can target what is driving the cancer,” said Poklepovic, who is a medical oncologist and member of the Developmental Therapeutics research program at Massey as well as assistant professor in the Division of Hematology, Oncology and Palliative Care at the Virginia Commonwealth University School of Medicine. “Additionally, mutations in pathways are not confined to one disease type. For example, you may see the same mutation show up in a stomach cancer as well as a breast cancer. So if you understand how that pathway works and how best to treat it, then you can apply that knowledge to both groups and benefit them both.”
Poklepovic is co-PI for the arm of NCI-MATCH that will examine the use of the drug sunitinib for c-Kit mutated cancers, which include gastrointestinal stromal tumors, melanoma and other systemic diseases. He will help oversee the respective protocol and prescribe this one of the trials’ nearly 25 available drugs attempting to “fix” the cell mechanism. He will also review patient data and recommend to the treating physicians any adjustments based on efficacy, or toxicity, and tumor response.
Regardless of geographic location of the patient or the managing PI, information will be shared on a secure network, allowing the researchers and physicians to communicate and share data as required.
“All options of this trial will be available at Massey. If a Massey patient has a mutation that is part of an arm of the trial led by a physician at another location, I will communicate with the appropriate PI, who will oversee the management of that aspect of the trial. These various treatments will be offered to patients in Virginia,” said Poklepovic.
Twenty-five percent of the cancers being studied on this trial are rare, low-frequency cancers. To get a representative number of patients, the trial is being conducted at cancer centers across the nation. The NCI anticipates the trial will open nationally in the summer 2015. For more information about the NCI-MATCH trial or any other clinical trials at Massey, visit www.massey.vcu.edu/clinical-trials/.
Written by: Massey Communications Office
Related News
Research
Robert A. Winn Clinical Investigator Pathway Program welcomes fourth cohort of medical student awardeesJul 8, 2025
Research
“We’re aiming for a cure.” Massey and VIMM researchers achieve potential breakthrough in brain cancer treatmentJun 24, 2025
Research
Massey researchers discover new genetic target that could shape the future of liver cancer treatmentJun 23, 2025

Get access to new, innovative care
Treatments in clinical trials may be more effective or have fewer side effects than the treatments that are currently available. With more than 200 studies for multiple types of cancers and cancer prevention, Massey supports a wide array of clinical trials.
Find a provider
Massey supports hundreds of top cancer specialists serving the needs of our patients. Massey’s medical team provides a wealth of expertise in cancer diagnosis, treatment, prevention and symptom management.
